Schrodinger Wave Equation
Quantum physics, one of the most fascinating branches of science, explains the behavior of microscopic particles like electrons and photons through concepts like the wave equation and the Schrödinger equation. At the core of quantum mechanics lies the Schrödinger Wave Equation, a fundamental equation that describes how quantum states evolve over time. Whether you’re preparing for competitive exams like JEE, BITSAT, or MHT-CET, understanding this equation is crucial.
What is the Schrödinger Wave Equation?
Proposed by Erwin Schrödinger in 1926, the Schrödinger Wave Equation provides a mathematical framework to determine the wave function () of a quantum system. The wave function encapsulates all possible information about a particle, including its position and momentum.
The time-dependent Schrödinger equation is written as:
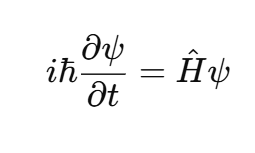
where:
- i = (√-1)imaginary unit
- ħ = reduced Planck’s constant (h/2π)
- Ĥ= Hamiltonian operator (total energy of the system)
- ψ = wave function of the system
- t= time
For steady-state systems, the time-independent Schrödinger equation is:
Ĥψ=Eψ
where represents the total energy of the quantum system.
Significance of the Schrödinger Equation
- Describes Quantum Behavior: Unlike classical mechanics, which gives precise values for position and momentum, quantum mechanics provides a probability distribution of a particle’s location.
- Foundation for Quantum Chemistry: It helps predict atomic and molecular structures, making it essential for understanding chemical bonding.
- Explains Particle-Wave Duality: The equation confirms that particles exhibit both wave-like and particle-like behavior.
Applications in Competitive Exams
For JEE preparation, BITSAT, and other engineering entrance exams, understanding the Schrödinger equation and key physics concepts for BITSAT, such as quantum mechanics for exams, can help with:
- Quantum Numbers and Orbitals: The equation helps derive quantum numbers that describe electron configurations in atoms.
- Tunneling Effect: A concept used in semiconductors and nanotechnology.
- Energy Quantization: Essential for solving problems on particle in a box, hydrogen atom models, and potential wells.
Final Thoughts
The Schrödinger Wave Equation is a cornerstone of modern physics, helping scientists and engineers develop lasers, transistors, and even quantum computers. As you prepare for your competitive exams, grasping the fundamentals of this equation can give you an edge.
Frequently Asked Questions on Schrodinger Wave Equation
Q1
What exactly is the Schrodinger wave equation?
The Schrodinger wave equation is a mathematical expression that describes the energy and position of an electron in space and time while accounting for the electron’s matter wave nature inside an atom.
Q2
List the applications of the Schrodinger wave equation.
It’s the foundation of wave mechanics.
It aids in the study of atomic structure.
It reflects the wave-like nature of matter.
It’s the foundation of wave mechanics.
Q3
What does the Schrodinger wave equation describe?
The Schrodinger wave equation describes the behaviour of matter waves.
For detailed explanations, solved examples, and interactive quizzes, explore Schrodingo’s best online courses for JEE and competitive exam preparation test series tailored for JEE, BITSAT, MHT-CET, and more!
